The main findings of this "iconoclastic" paper can be summarized in the following:
1. There are discrepancies between the nucleosome maps obtained through MNase sequencing and Chemical Mapping. This comes as an additional level of complexity given that a number of works (see examples here and here) have pointed out the discrepancy between nucleosome positions in vitro and in vivo. This becomes even more complex given that:
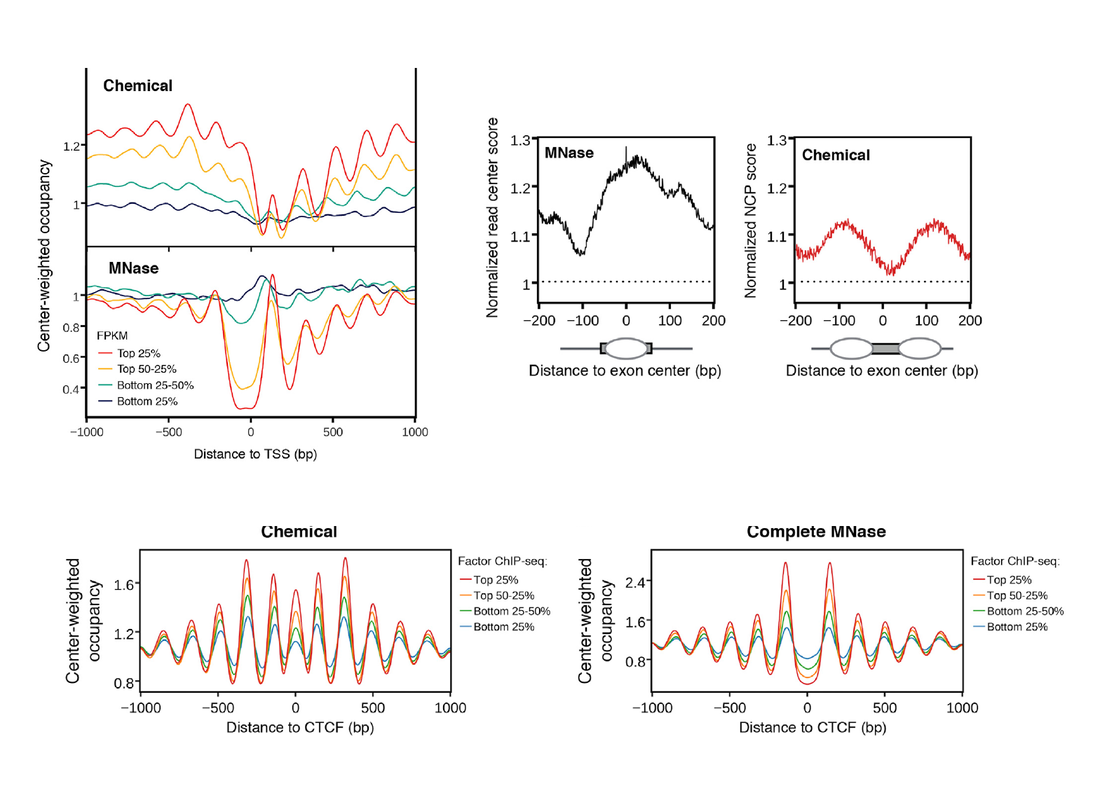
a. The authors find positioned nucleosomes exactly where other methods have repeatedly reported nucleosome-free regions such as upstream of transcription start sites, at CTCF binding sites and at the binding sites of pioneer transcriptional regulators of differentiationsuch as Oct4, Sox2 and Nanog. These are all well-reported cases of nucleosome-free regions being key genomic elements of regulation through chromatin (examples here for differentiation TF and here for CTCF). Last but not least, the authors observe a lack of nucleosomes positioned within exons, and instead find them at exon-intron junctions, contrary to what has been widely reported by a number of studies (e.g. here) among which ours was one of the first.
2. The authors attribute these marked discrepancies to inherent biases of the MNase sequencing protocol. This is valid, as a number of works, have in the past reported that digestion timing influences MNase output, as "fragile nucleosomes" (that is nucleosomes that are less stably bound to the the DNA) are more prone to digestion and are thus lost from the sequencing signal. The authors perform a number of convincing analyses to show that the nucleosomes they detect at TSS and pioneer factors binding sites (but not in exon/intron junctions) are indeed such "fragile nucleosomes".
3. Last but not least, the paper shows a concise nucleosome profile clustering of TSS-regions that is revealing of great variability in the positioning patterns. This is one of the most interesting parts of this study, since such variability can account for the rather complex (and often inconclusive and odd) mean patterns of nucleosome positioning when all genes are pooled together. This finding is reminiscent of similar variability observed among yeast genes (see e.g. here) and even between yeast species such as S. cerevisiae and S. pombe.
The paper is solid methodologically (as it should be, after all it is a Cell Highlight Paper) and one cannot argue about the presented results. Fragile nucleosomes may be indeed picked up by this method but the problem that arises is conceptual rather than factual. A recurrent theme on the phenomenon of nucleosome positioning is its statistical nature, and many (including ourselves here, but most conclusively Andy Fire's lab here) have shown that only a small portion of nucleosomes are well- and reproducibly positioned in a homogeneous cell population. This means that only around 1/10 nucleosomes are actually a) guided by the underlying sequence through specific compositional constraints and b) appear to "matter" in terms of mechanistic aspects related to other functions. Such nucleosomes are precisely the ones found downstream of TSS, on either side of well-defined nucleosome-free regions and within exons, which is striking since these are exactly the points of discrepancy between MNase and the Chemical method presented by Voong et al. What is going on here? Is this a case of a new methodological protocol turning the tables? Is everything we knew about nucleosome positioning wrong? Or could it be that chemical mapping somehow "fixes" nucleosomes on positions that are not functionally relevant? The case of exons is rather odd, since it appears that the two methods yield almost mutually exclusive patterns (see adapted Figure below). Is MNase "pushing" nucleosomes to the center of the exon, or is Chemical mapping fixing them towards the boundaries?
My hunch tells me that we should not rush into discrediting MNase maps yet and this not so much due to the fact that I have been working with those for most of my academic years, but because of a very sound theoretical argument, with evolutionary implications. A number of theoretical models that predict nucleosome positioning have consistently found NFR and well-positioned nucleosomes to coincide with those obtained through MNase sequencing, which means that if we are to "ask" the sequence (at least with the models we have so far) it will tell us that nucleosomes tend indeed to avoid promoter regions, exon-intron junctions and TF-binding sites. This is not entirely contradicted in this work by Voong et al., since they also admit that the "new" nucleosomes belong to the "fragile" counterpart and are thus expected to be less strongly bound. From another point of view, it would be extremely interesting to look at the "fragile nucleosomes" that are picked up by the Chemical mapping and see what predictive models tell us about some of those. I promise to come back to this point, hopefully with some of our own analyses.
Untill then, beware of maps even bearing nucleosomes.
 RSS Feed
RSS Feed
